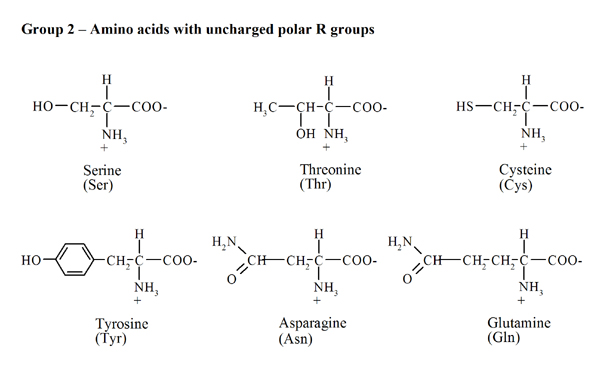
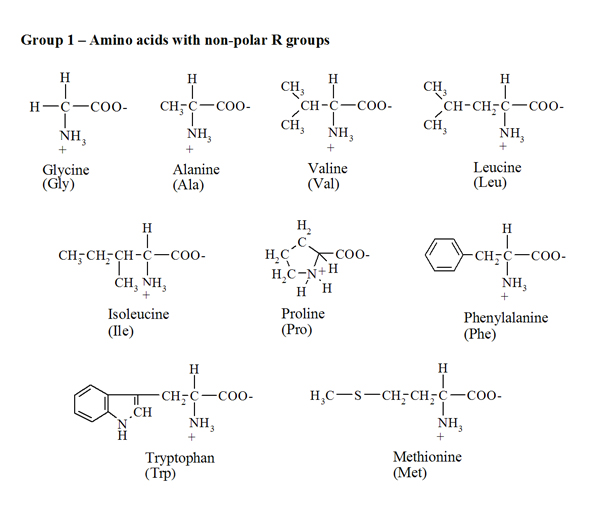
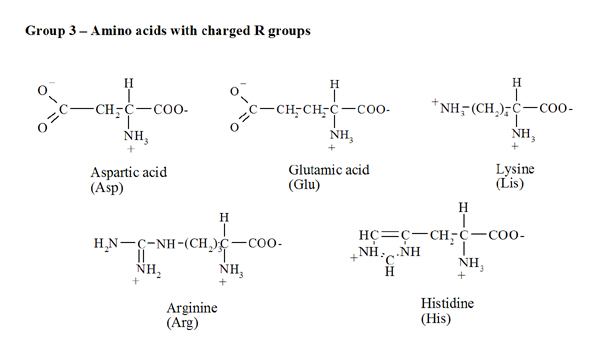
Amino acidsProtein molecules consist of a large number of monomers connected to one another. The amino acids are precisely these monomers or building blocks, carrying out an important role in the living organism's metabolism since amino acids are essential to keep the nitrogen balance and to promote growth. Moreover, amino acids have a strong influence on the nutritive value of foods, as direct contributors to taste and as precursors of several compounds that are formed during preparation, storage and cooking. The proteins are hydrolysed into twenty different amino acids, nineteen of which are a a-amino acids - this means that the amino group (NH2) is bound to the carbon atom adjacent to the carboxyl group. The general formula is RCH(NH2)COOH, in which the radical R (side chain) ranges from a simple hydrogen atom (for glycine) to more complex aliphatic, aromatic or heterocyclic groups. The only exception to this general formula is proline since the NH2 group is included in a five-carbon cyclic structure. Every amino acid's name is abbreviated by a three-letter code - based on the first three letters of their names (Fig. 1).The specific side chain R of each amino acid affects their physical and chemical properties and, consequently, the properties of proteins. According to R's polarity (Fig. 1) it is possible to group the amino acids into four classes: (i) uncharged non-polar side chain (alanine, glycine, valine, leucine, isoleucine, proline, phenylalanine, tryptophan and methionine), (ii) uncharged polar side chain (serine, threonine, cysteine, tyrosine, asparagine and glutamine), (iii) charged side chain (positive charge: lysine, arginine and histidine; negative charge: aspartic and glutamic acids). |
||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig.1. Chemical structure, name and three-letter code for the various amino acids. |
||||||||||||||||||||||||||||||||||||||||||||||||||||
From a nutritional point of view, amino acids are classified into two groups: essential (amino acids that humans are unable to synthesize and, therefore, must be obtained through their diet) - valine, leucine, isoleucine, phenylalanine, tryptophan, methionine, histidine, threonine, lysine and arginine (semi-essential) - and nonessential - glycine, alanine, proline, serine, cysteine, tyrosine, asparagine, glutamine, aspartic and glutamic acids. The nonessential amino acids are as important as the essential ones, being irreplaceable in the organism's physiological processes, nonetheless humans can live without their presence in the diet. For instance, cysteine and tyrosine are indispensable to adult humans but not essential since the organism produces the first from methionine and the second from phenylalanine.Similarly to proteins present in milk, eggs and meat, seafood proteins have high biological value because they contain all amino acids essential for human nutrition (Table I). |
||||||||||||||||||||||||||||||||||||||||||||||||||||
Table I. Average levels of the essential amino acids (%) in proteins of different origins (seafood, milk, beef and egg). |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
If you want to know more, see:Belitz, H.-D.; Groseh, W., 1985. Química de los alimentos. Editorial Acribia. S.A., Zaragoza. 813p.Ferreira, F.G., 1983. Nutrição Humana. Fundação Calouste Gulbenkian, Lisboa. 1291 p. |
||||||||||||||||||||||||||||||||||||||||||||||||||||